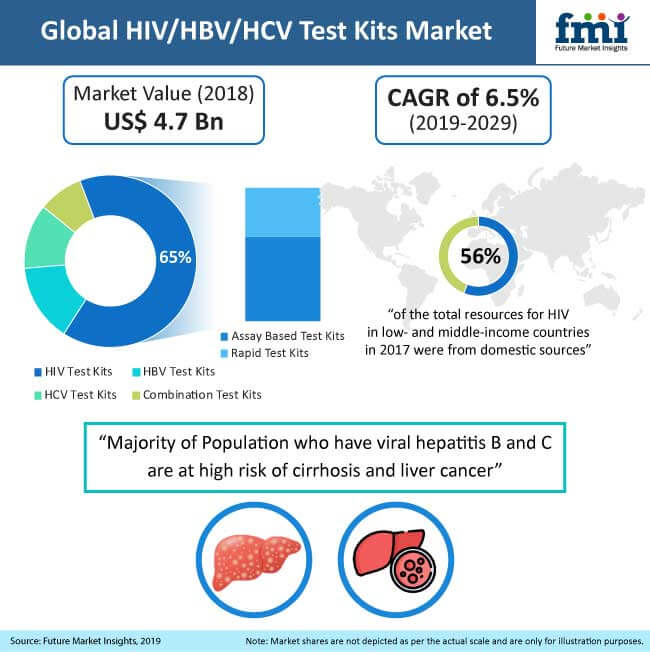
The increasing burden of HIV and viral hepatitis infections is estimated to boost the HIV/HBV/HCV test kits market. Assay-based test kits are expected to contribute a large market share as these are the first line of tests prescribed to patients. Moreover, in most of the developing regions, healthcare professionals and patients rely on assay-based testing results. Increase in the number of initiatives undertaken by government organisations and NGOs to raise awareness regarding the available testing materials and treatment options is expected to boost the growth of the HIV/HBV/HCV test kits market over the forecast period.
The rising adoption of point-of-care testing is expected to boost the demand for rapid test kits and is anticipated to grow at a high rate during the forecast years. According to the latest report published by the FMI, the global HIV/HBV/HCV test kits market, in terms of market value, is expected to account for US$ 9.4 Bn by the forecast year 2029. The report further projects that the HIV/HBV/HCV test kits market will grow at an approximate CAGR of 7.0% during the forecast period 2019-2029.
Request Sample Report @ https://www.futuremarketinsights.com/reports/sample/rep-gb-9814
R&D, Clinical Trials, & Government Initiatives Collectively Provide Impetus
Over the past decades, HIV/HBV/HCV test kits have gained significant popularity and an upsurge in demand across the world. According to the United Nations Programme on HIV/AIDS (UNAIDS), the global prevalence of HIV was around 0.8% among adults in 2017, of which 75% of the people are aware of their HIV status. Of these, 79% are receiving treatment. The demand for combination rapid test kits is expected to grow at a high rate, which is estimated to contribute a high revenue share to the HIV/HBV/HCV test kits market over the forecast years. Combination rapid test kits provide the result for HIV, HBV, and HCV infection together by using one sample within 20 min to half an hour. This factor is expected to play an instrumental role in driving the growth of the HIV/HBV/HCV test kits market.
Lack of awareness regarding the available treatment options and the asymptomatic nature of infections is expected to hamper the growth of the HIV/HBV/HCV test kits market. In 2015, an estimated 257 Mn people were living with chronic hepatitis B infection, and 71 Mn people with chronic hepatitis C infection, due to lack of awareness regarding the symptoms. Hence, majority of the population remained underserved. The East Asia and Africa HIV/HBV/HCV test kits markets are expected to witness significant growth in the patient pool due to the high prevalence of infections in these regions, coupled with lack of awareness, poor healthcare infrastructure, and treatment facilities.
However, rising R&D and clinical trials, steps taken to reduce the cost of treatment, and initiatives by governments & NGOs to spread awareness and provide free check-up centres are some of the factors boosting the growth of the HIV/HBV/HCV test kits market. The East Asia HIV/HBV/HCV test kits market is estimated to create an approximate incremental $ opportunity worth US$ 7.7 Bn between 2019 and 2029.
Request to View Methodology @ https://www.futuremarketinsights.com/askus/rep-gb-9814
The global HIV/HBV/HCV test kits market has been segmented by kit types, sample type, end user, and regions. Furthermore, the test kits type of HIV/HBV/HCV test kits market has been segmented into assay-based test kits, rapid test kits, and combination rapid test kits, which are used for the detection of HIV and viral hepatitis B & C infections. The sample type segment of the HIV/HBV/HCV test kits market is segmented into blood, urine, and saliva. Blood sample type is expected to hold a large share during the forecast period. In terms of end user, HIV/HBV/HCV test kits are segmented into hospitals, clinics, diagnostic laboratories, government organisations & NGOs, and others.
The report tracks some of the key companies operating in the HIV/HBV/HCV test kits market, such as Hoffmann-La Roche Ltd., Abbott Laboratories, bioMérieux SA, Bio-Rad Laboratories Inc., QIAGEN, Hologic Inc., Meridian Bioscience, Inc., Maternova, Siemens AG, and Creative Diagnostics.
No comments:
Post a Comment